Tel:0512-66958483
Email:engforlife-mkt@efl-tech.com

試劑/耗材
Reagents / Consumables

Microneedle Preparation Solutions
This solution includes materials for microneedle preparation, the Microneedle Molds, the Vacuum Defoaming Device, the Vacuum Liquid Filler, and the Microneedle Drying Kit.
Salesperson:Panpan Wang(EFL)
Phone:15335258233
Email:engforlife-mkt@efl-tech.com
Foreign distributor:ANR Technologies Pte Ltd
Compared with traditional metal or silicon-based microneedles, hydrogel-based microneedles offer the following significant advantages: 1) High drug loading capacity and controllable drug release; 2) Needle materials composed of polymer biomaterials with excellent biocompatibility, avoiding potential skin damage caused by needle tip breakage; 3) Cross-linked polymer biomaterials enable microneedles to swell rather than dissolve subcutaneously, leading to more sustained drug release.
However, the preparation of hydrogel-based microneedles also faces multiple challenges, such as high solution viscosity, difficulty in liquid injection and defoaming, the microneedle easy to wrinkling, uneven substrates, irregular needle shapes, and low needle tip strength, etc.
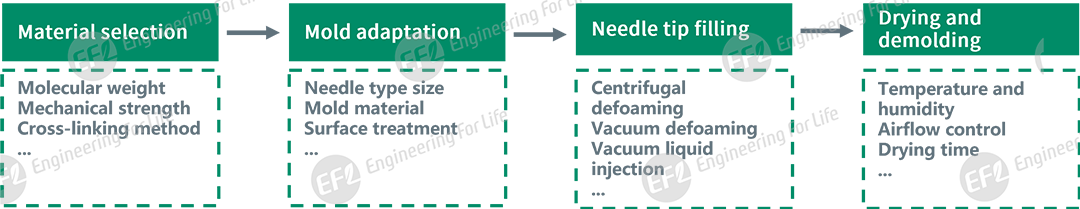
To address these issues and streamline microneedle preparation, EFL has developed a comprehensive solution covering key processes: material selection, mold adaptation, needle tip filling, and drying and demolding.
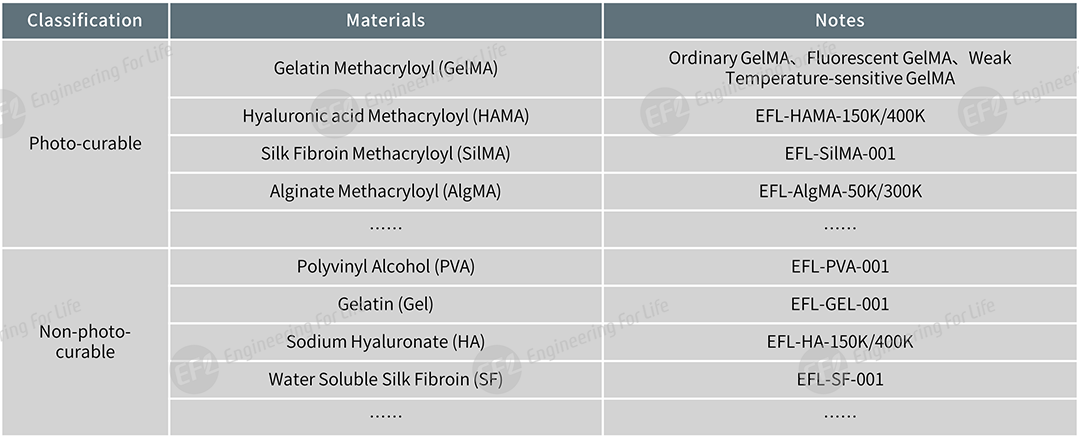
EFL provides both photo-curable and non-photo-curable biomaterials for microneedle preparation.
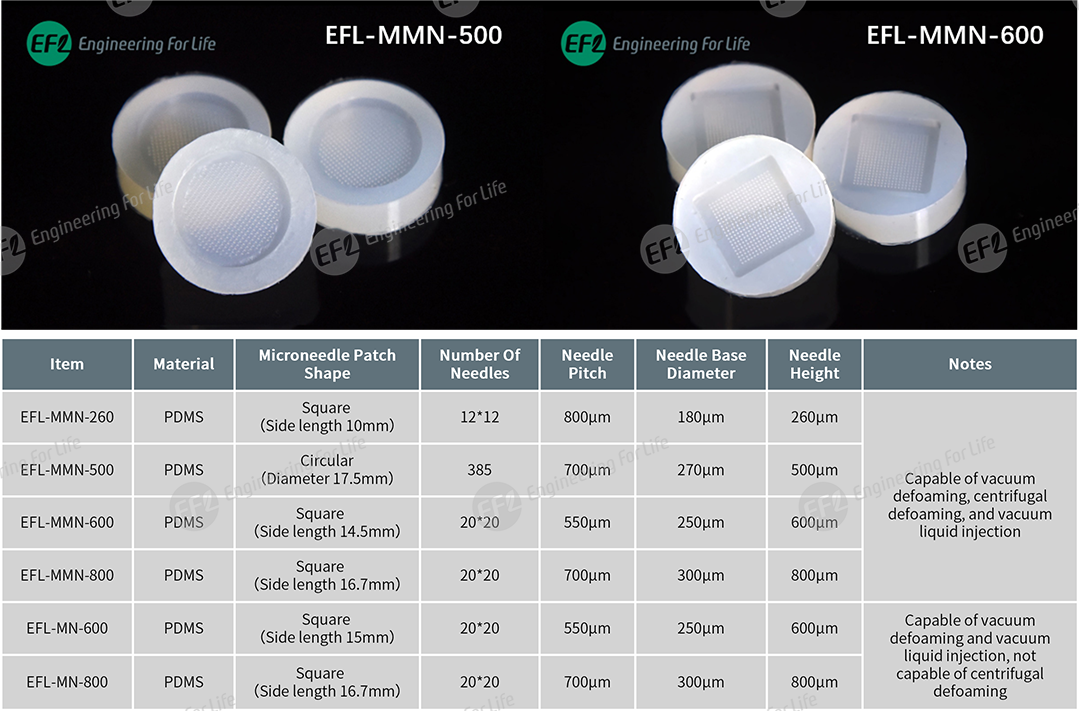
Based on long-term communication and feedback from users, EFL has optimized commonly used specifications of microneedle molds, as detailed below:
Option 1: Vacuum Defoaming Process (compatible with the Vacuum Defoaming Device EFL-VDM-001)
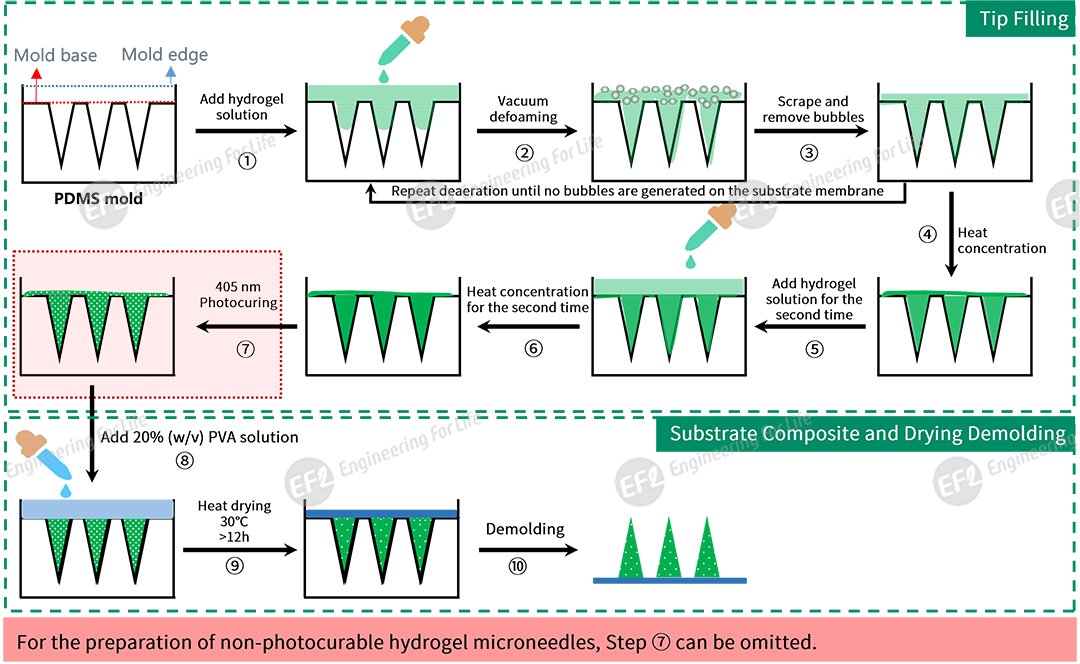

Option 2: Vacuum Liquid Injection Process (compatible with the Vacuum Liquid Filler EFL-VLF100)
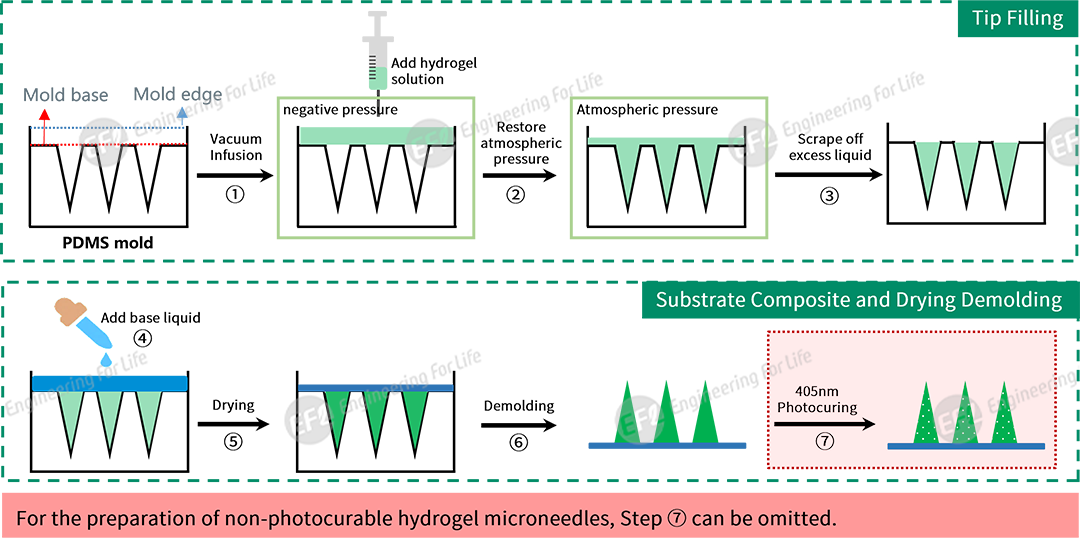

The drying process significantly impacts the morphology and performance of microneedles. Improper control may lead to issues such as needle tip deformation, separation of the substrate and needle tip, hollow substrates, wrinkled and uneven substrates, and drug inactivation. Leveraging extensive process research experience, EFL has developed a Microneedle Drying Kit (EFL-VLF-006) that minimizes interference from external airflow and humidity. This kit enables microneedle drying under low-temperature, room-temperature, or high-temperature conditions, yielding well-shaped microneedle patches.

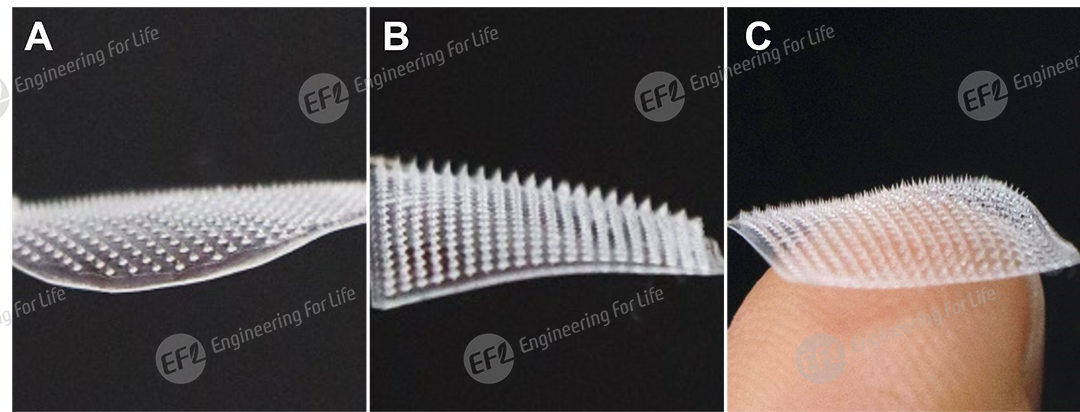
Fig 1. Images of HAMA photo-curable swellable hydrogel microneedles (A: needle height 500μm, basal diameter 270μm, patch diameter 17.5mm; B,C: needle height 600μm, basal diameter 270μm, patch size 14.5mm×14.5mm)
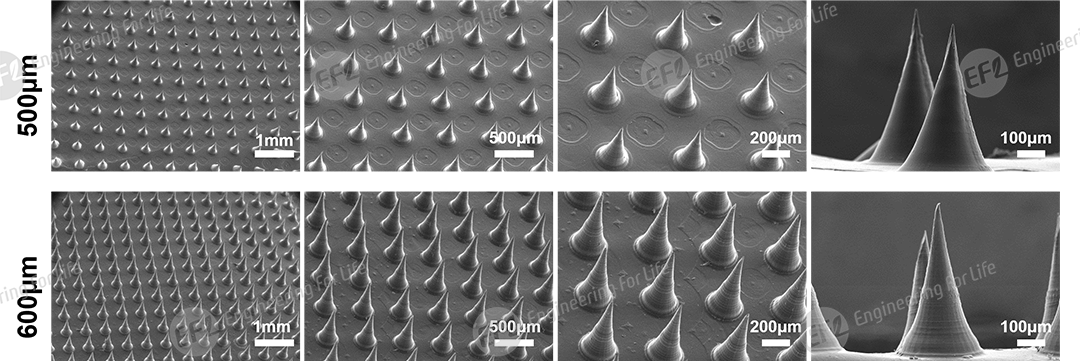
Fig 2. SEM images of microneedles with needle heights of 500μm and 600μm
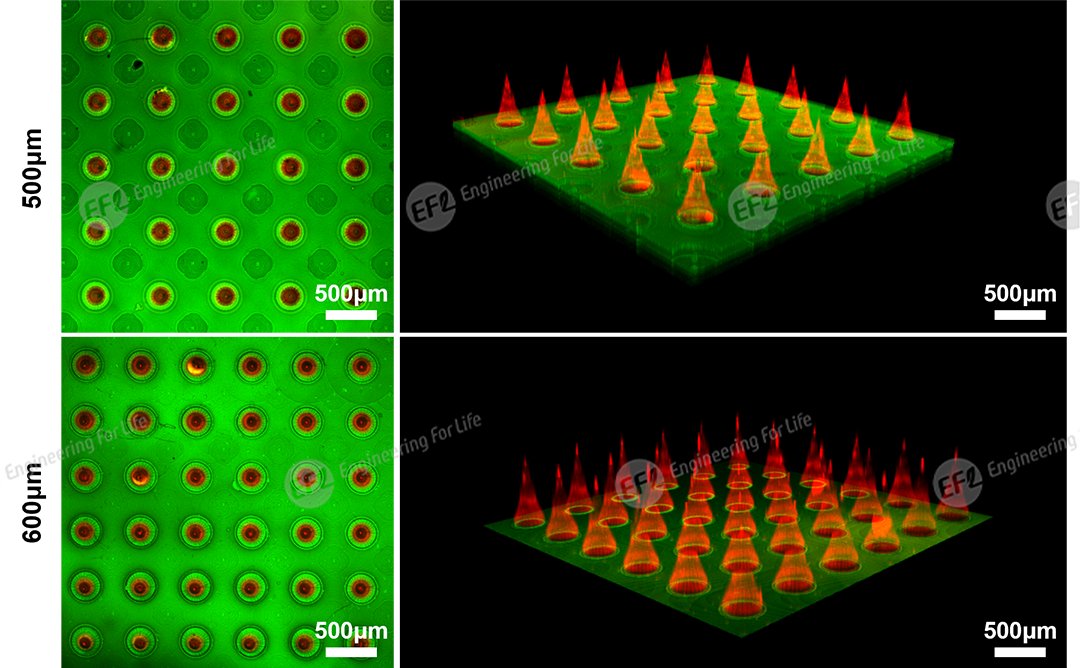
Fig 3. CLSM images of microneedles with needle heights of 500μm and 600μm (needle tips labeled with rhodamine B, substrate labeled with fluorescein)
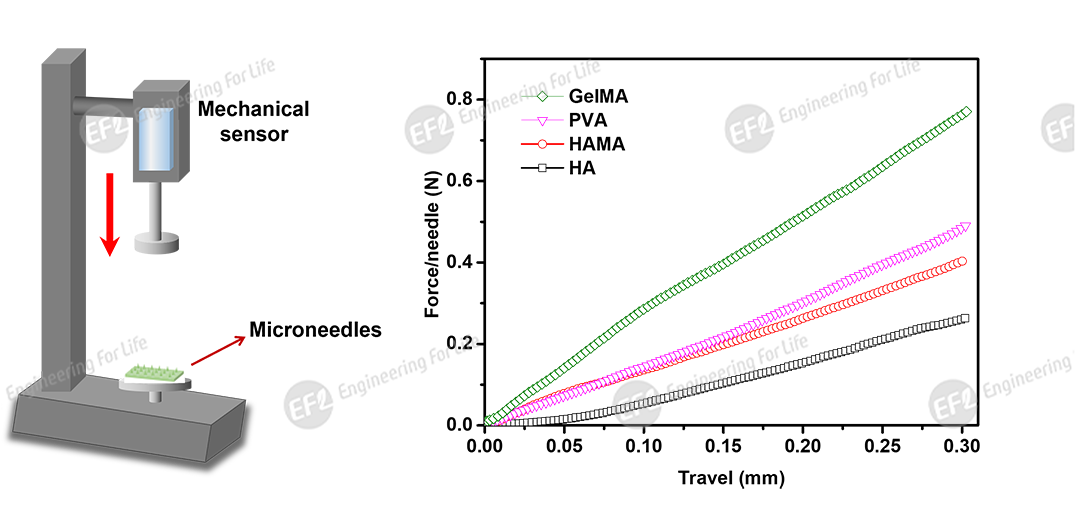
Fig 4. Force-displacement curves of different microneedles